Solid calcium reacts with water to produce aqueous calcium hydroxide and hydrogen gas. Calcium hydride reacts with water to form calcium hydroxide aqueous and hydrogen gas.

Q Write A Chemical Equation For This Caclcium Water Calcium Hydroxide Oxygen Chemistry 12726347 Meritnation Com
When solid copper reacts with aqueous silver nitrate the products are.
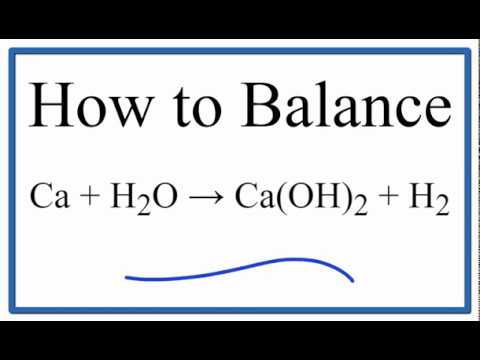
. The balanced equation for this is CaH2 2H2O ---. This is a displacement reaction as calcium dispalced hydrogen from water to form calcium hydroxide. Reaction of Elemental Calcium with Water is as follow Ca 2 H₂O Ca OH₂ H₂.
C a 2 H 2 O C a O H 2 H 2. Compute answers using Wolframs breakthrough technology knowledgebase relied on by millions of students professionals. -The molar mass of hydrogen is 2016 gmol.
Solve any question of Chemical Reactions and Equations with-. The second step is the formation of solid calcium hydroxide as the only product from the reaction of the solid calcium oxide with liquid water. Calcium hydride reacts with water to form calcium hydroxide and hydrogen gas.
The balanced chemical reaction. The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide. For math science nutrition history geography engineering mathematics.
Using the coefficients to the left of each chemical there is a total of six hydrogen atoms six hydroxide molecules three calcium atoms and two phosphate molecules on each side of the equation. 080mol CaH2 s and 152 mol H2O l. All atoms are balanced in both directions.
Part - a The balanced chemical reac. Calcium hydride hydrogen calcium hydroxide water. B How many grams of calcium hydride are needed to form 4500 g of hydrogen.
Calcium hydroxide is relatively insoluble in water with a solubility product K sp of 55 Ã 10 Ã6. Calcium hydroxide water calcium hydride hydrogen. At room temperature calcium reacts with oxygen forming a thin layer of CaO that protects the metal from further oxidation.
CID 1118 Sulfuric acid CID 5460341 Calcium Dates. The calcium reacts with water to produce calcium hydroxide and hydrogen gas. Calcium Hydroxide And Hydrochloric Acid Chemical Equation How To Write The Net Ionic Equation For Ca Oh 2 Ch3cooh Ca Ch3coo 2 H2o Youtube Calcium phosphate is a calcium salt of phosphoric acid with a chemical formula ca 3 po 4 2.
Ca 2 H2O. After a second or so the calcium metal begins to bubble vigorously as it reacts with the water producing hydrogen gas and a cloudy white precipitate of calcium hydroxide. Write a balanced chemical equation for each step of the process.
The given balanced chemical equation is as follows. Ca s 2H 2 O l Ca OH 2 aq H 2 g In the following demonstration a chunk of calcium metal is dropped into a beaker of distilled water. Reaction of calcium with air.
Calcium hydride water calcium hydroxide hydrogen. Write the chemical reaction and balance the equation. View the full answer.
Calcium hydride reacts with water to form calcium hydroxide and hydrogen gas. Calcium hydride reacts with water to form calcium hydroxide and hydrogen gas. Ca OH2 H2 The required mass of H2 is 0026 g Explanation.
Calcium metal reacts with water to produce aqueous calcium hydroxide and hydrogen gas. - In the above chemical reaction one mole of calcium hydride reacts with two moles of water and forms one mole of calcium hydroxide and two moles of hydrogen as the products. 2 Ca s O 2 g 2 CaO s.
MgH 2 reacts readily with water to form hydrogen gas. CaOH2 2H2 a How many grams of calcium hydride are needed to form 4500 g of hydrogen. What is the equation for the reaction between oxygen and calcium hydroxide.
Hydrochloric acid calcium hydroxide - calcium chloride water. MgH 2 2 H 2 O Ââ 2 H 2 Mg OH 2. Calcium can be ignited and will when burning react with both oxygen and nitrogen forming calcium oxide CaO and calcium nitride Ca 3 N 2.
CaH 2 O 8 S 2. 2 H3PO4 3 Ca OH2 6 H2O Ca3 PO42. Calcium hydroxide and hydrogen gas are produced by the reaction of calcium hydride and water.
No reaction between calcium. Here 1 mole of calcium completely reacts with 2 moles of water and forms one mole of calcium hydroxide and one mole of hydrogen as gas. C a H 2 2 H 2 O C a O H 2 2 H 2.
Calcium metal reacts with water to form calcium hydroxide and hydrogen gas. Extended Keyboard Examples Upload Random. The entire chemical equation is represented as.
Cas 2H2Ol CaOH2aq H2g Write balanced equation. Calcium hydride CaH2 reacts with water to form hydrogen gas. In both cases corresponding Hydroxides along with Hydrogen gas is produced.
CaH2 s2H2O lCa OH2 aq2H2 g This reaction is sometimes used to inflate life rafts weather balloons and the like where a simple compact means of generating H2 is desired. When Sodium Metal is reacted with Water it produces NaOH and eliminates Hydrogen gas as shown in following equation 2 Na 2 H₂O 2 NaOH H₂. Thymol blue is used as the indicator for a titration.
A Write a balanced chemical equation for the reaction. What information do we obtain from the titration to help us determine the formula for calcium hydroxide. You will titrate the solution with HCl aq to determine the chemical formula for calcium hydroxide.
O CaOH 2. Calcium hydroxide hydrogen calcium hydride water.

Ca H2o Ca Oh 2 H2 Balanced Equation Calcium Water Calcium Hydroxide Hydrogen Balanced Equation Youtube

Write Complate Balanced Equations For The Following Reactions A Calcium Solid Water Liquid Youtube
0 Comments